Over the past few years alone, “digital healthcare has grown exponentially” and “the amount of data collected by the industry has increased by almost 50% every year.”[1] With advances in technology—its wide use and global accessibility—data collection and its analysis is fast becoming one of the world’s most valuable assets. Mapping the world around us in real-time, informing our present—and ultimately, deciding our future (for the complete article read Embracing data collection for success).
The EU’s Medical Devices Regulation 2017/745 (MDR)—in effect from May 2020—makes data collection and clinical evidence a regulatory requirement to obtain a CE mark to access the European market.
The MDR particularly emphasises the importance of the Clinical Evaluation Report (CER), specifying that clinical data must be “scientifically valid, reliable and robust” whilst also ensuring that the “rights, safety, dignity and well-being of the subjects participating in a clinical investigation are protected and prevail over all other interests.”[2]
Whilst marking a significant gear change in the industry, this shift will improve patient safety and increase market transparency, creating new opportunities for manufactures and protecting existing products on the market.
Manufacturers that already have this clinical data to hand will find that they not only get to keep their products on the European market, but also gain the advantage of a competitive edge (in part, due to the expense and considerable length of time clinical studies take to complete).
The data collected for Eudamed will allow these companies to track and analyse the performance of devices on the market and mine for information that will reveal patterns and opportunities in the market that manufactures can then act on.
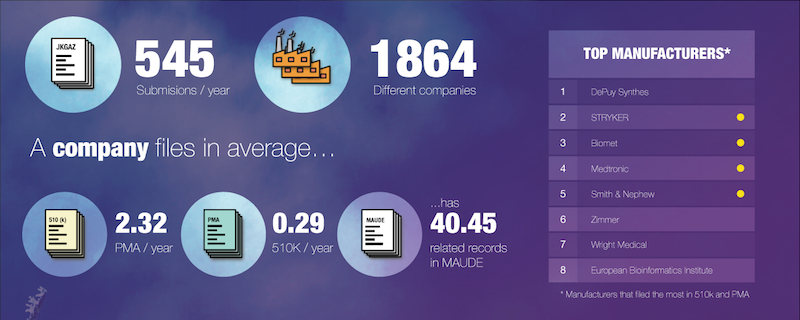
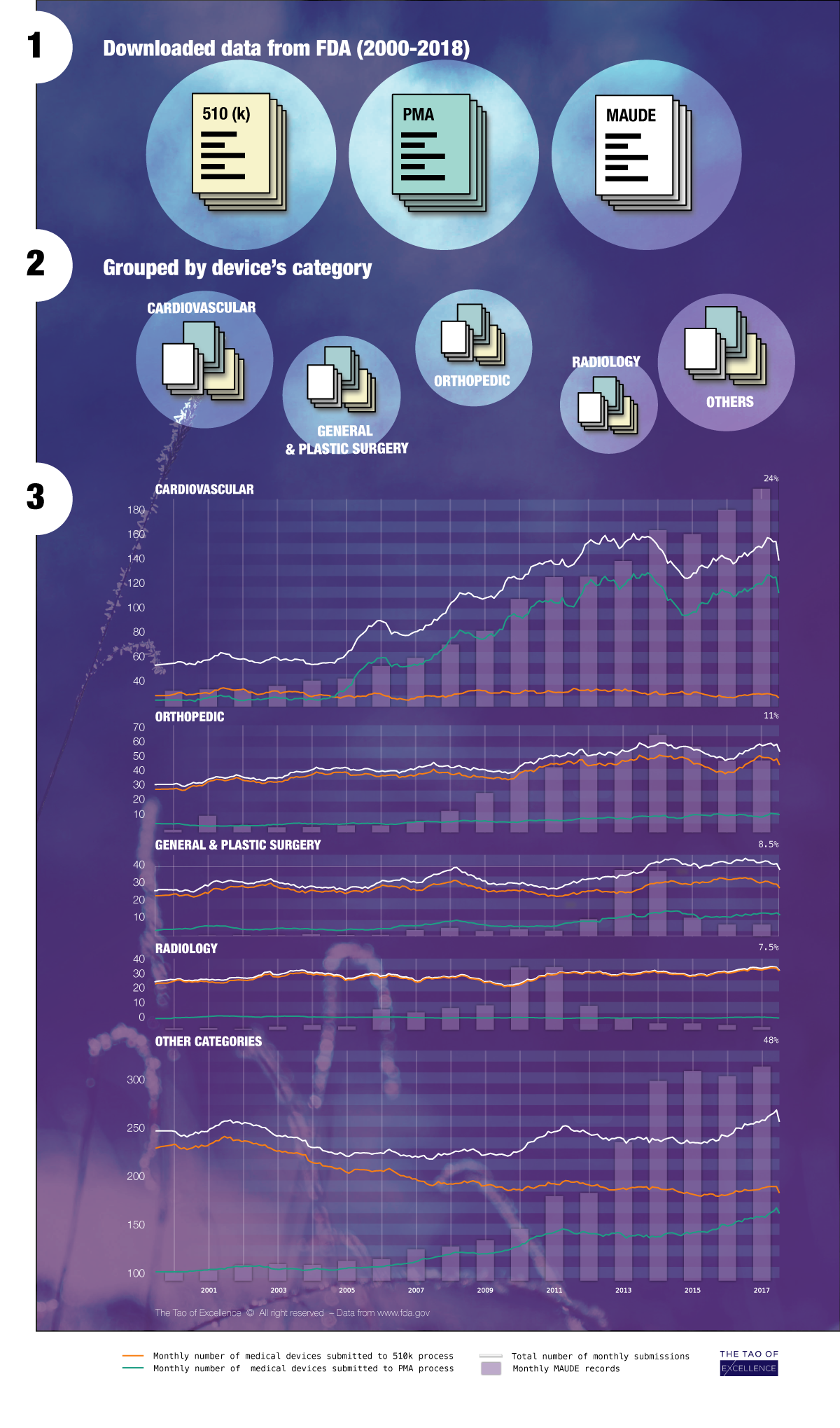
The following infographic is an example of such an analysis. Using public data from the FDA’s database, this infographic details the wealth of market information that will be increasingly accessible to manufactures. From large companies to SMEs, businesses will be able to track the market by category, premarket submissions and more, whilst including contextual factors such as the financial market and regulatory updates.
 The Tao of Excellence has a team of experts in data collection, analysis and strategy. Our specialists can give you an evaluation of the current status of your company’s data and its regulatory requirements, an analysis your competitors and the wider market, and a strategic market report that can help you to develop and secure the true potential of your product portfolio. Contact us today for a consultation or visit our website to learn more about how we can assist your organization.
The Tao of Excellence has a team of experts in data collection, analysis and strategy. Our specialists can give you an evaluation of the current status of your company’s data and its regulatory requirements, an analysis your competitors and the wider market, and a strategic market report that can help you to develop and secure the true potential of your product portfolio. Contact us today for a consultation or visit our website to learn more about how we can assist your organization.
 Jasminka Roth
Jasminka Roth
Founder and Director of The Tao of Excellence
Phone
+41 52 685 51 65
Email
meetus@taoexcellence.ch
Did you like this article? Follow us on LinkedIn and Twitter!
[1] Novartis Press Release, 12 July 2018: https://sciencebusiness.net/network-news/novartis-drug-development-gets-big-data-analytics-boost
[2] Regulation (Eu) 2017/745 of the European Parliament and of the Council, 5 April 2017: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0745