Access our services for support with your MDR, IVDR, MDSAP compliance and medical devices certification, business intelligence, audit preparation and the improvement and digitalization of your processes and procedures. From data and gap analysis to securing a successful regulatory strategy and implementation of a high-performance and sustainable QMS (Quality Management System), The Tao of Excellence has the specialist expertise to support your business development for the future.
Services
Shop
-

Importer Training
For Swiss Importers
Read more -

Regulatory document matrix for medical devices
For the EU and Swiss market
100 CHF plus VATAdd to cart -




Checklist for label
for medical devices in EU and Switzerland.
175 CHF plus VATSelect options -





CH-REP Manual for Switzerland
Conformity without a certified QMS.
2'000 CHF plus VATSelect options -





Importer Manual for Switzerland
Conformity without a certified QMS.
2'000 CHF plus VATSelect options -








EU-Konformitätserklärung (DoC)
Checkliste zur Prüfung der DoC.
50 CHF plus VATAdd to cart -


IVDR Review
Support checking your SOPs against the IVDR.
Read more -








IVDR Risk Management
Gap analysis tool for risk management compliance.
175 CHF plus VATAdd to cart -








MDR UDI
Gap analysis tool for UDI compliance.
155 CHF plus VATAdd to cart -






Process Improvement 2
Level 2. Certified Six Sigma Green Belt Course.
5'750 CHF plus VATAdd to cart -






Process Improvement 1
Level 1. Certified Six Sigma Yellow Belt Course.
1'420 CHF plus VATAdd to cart -








Post Market Surveillance
Gap analysis tool for PMS compliance.
155 CHF plus VATAdd to cart -


RA / QA Membership
Regulatory and quality management support.
Read more -




DOE Course
Learn to master design of experiments.
810 CHF plus VATAdd to cart -








MDR Clinical Evaluation
Gap analysis tool for GCP compliance.
190 CHF plus VATAdd to cart -


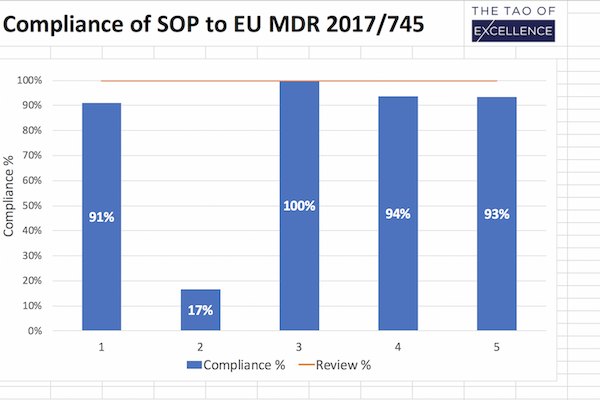
MDR Review
Support checking your SOPs against the MDR.
Read more -






Work with a Notified Body
Workshop on how to work with a Notified Body.
200 CHF plus VATAdd to cart -






Contact a Notified Body
Workshop on finding and contacting a Notified Body.
200 CHF plus VATAdd to cart -






Select a Notified Body
Workshop on how to select a Notified Body.
200 CHF plus VATAdd to cart -









MDR Risk Management
Gap analysis tool for risk management compliance.
175 CHF plus VATAdd to cart -









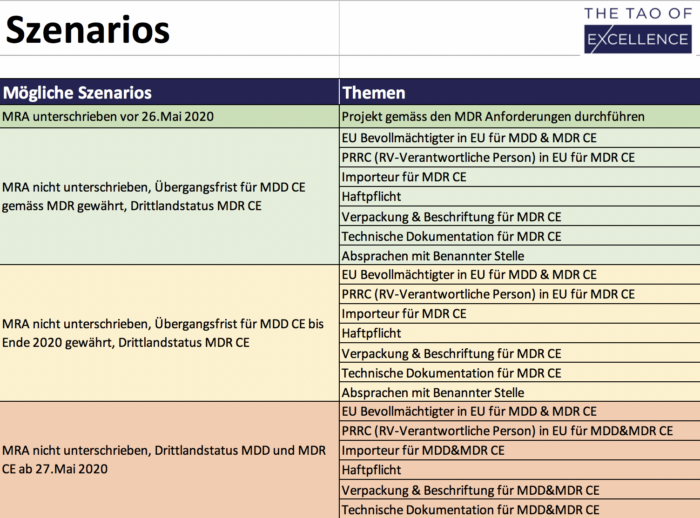
MRA Solutions Finder
Tool to optimize preparation for a missing MRA.
600 CHF plus VATAdd to cart -






ISO 13485 Starter Kit
The QMS for your medical device.
4'150 CHF plus VATAdd to cart -






ISO 9001 Starter Kit
Set up a new quality management system.
3'400 CHF plus VATAdd to cart








