MRA Solutions Finder Tool to optimize preparation for a missing MRA.
600 CHF plus VAT
- Downloadable excel file that can calculate and prioritise the right solutions for your business.
- A final regulatory strategy prioritised by price, time and site-specific insights.
- 3-hour consultation to optimise your use of this tool and answer regulatory questions.
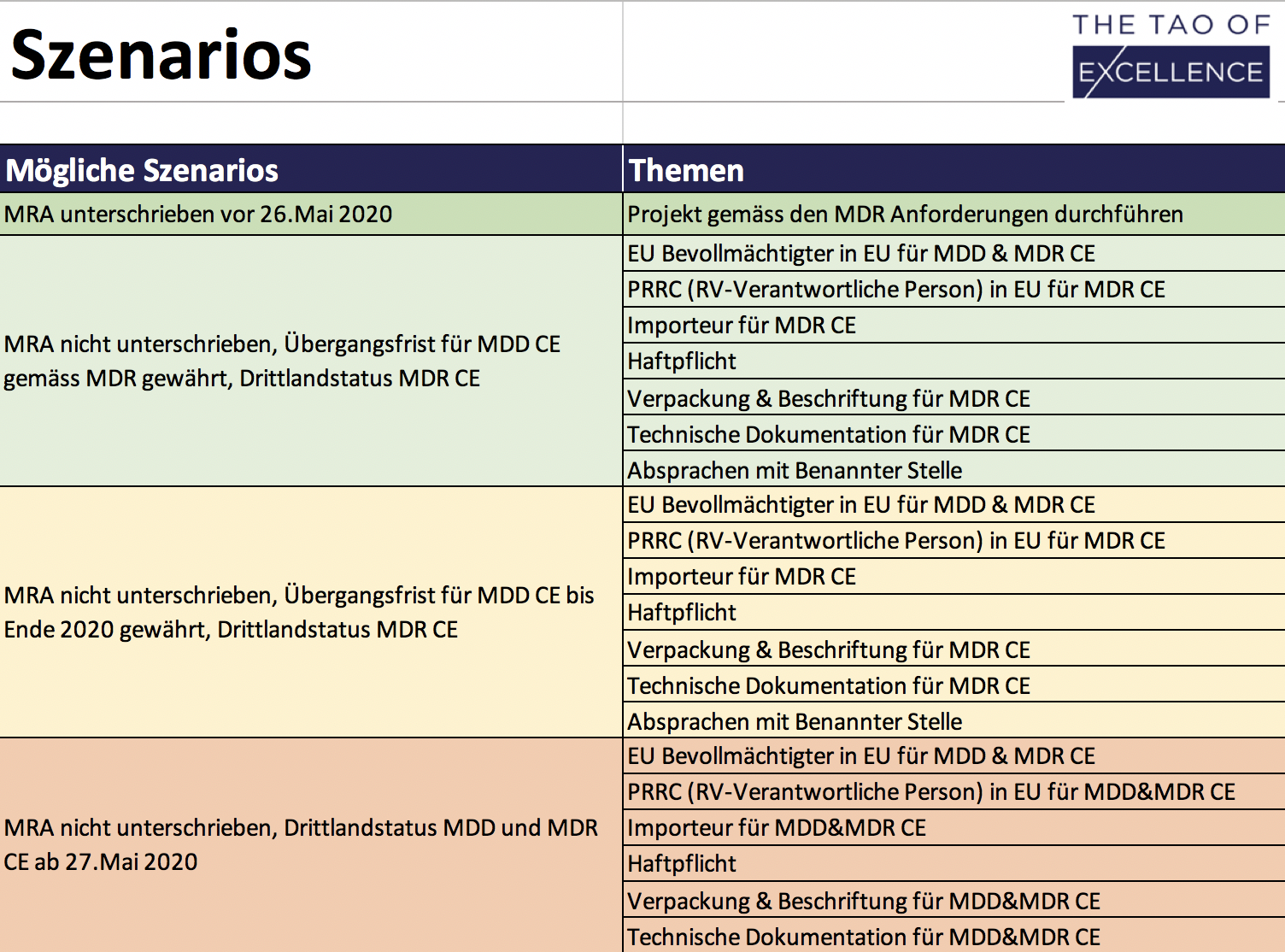
- Clearly defined MRA / MDR scenarios with the regulatory requirements for each area.
- A checklist of all the solutions and the steps to consider.
- Inbuilt formula to calculate which tasks to complete first.
- Insight from your team integrated into the formula to prioritise tasks.
Description
Medical device manufacturers in Switzerland must now prepare to face ‘third country’ requirements in the EU. The Swiss/EU Mutual Recognition Agreement (MRA) will remain unsigned before the application of the new Medical Device Regulation (MDR) 2017/745, thereby removing the free movement of Swiss medical devices in the EU. This MRA Solutions Finder is a downloadable Excel tool that can find the right solutions for your business, calculate the costs involved and create a prioritised list of actionable tasks for an up-to-date regulatory strategy. The MRA Solutions Finder evaluates solutions by price, time and with the option to integrate site-specific insight from members of your team. This package includes a 3-hour consultation to optimise this tool’s performance, guide you through the evaluation process and answer your regulatory questions.
Contact Form
You may also like…
-

ISO 13485 Starter Kit
The QMS for your medical device.
4'150 CHF plus VATAdd to cart -

RA / QA Membership
Regulatory and quality management support.
Read more -


MDR Risk Management
Gap analysis tool for risk management compliance.
175 CHF plus VATAdd to cart -


MDR Review
Support checking your SOPs against the MDR.
Read more