MRA
Showing all 9 results
-
CH-REP Manual for Switzerland
Conformity without a certified QMS.
2'000 CHF plus VATSelect options -

Checklist for label
for medical devices in EU and Switzerland.
175 CHF plus VATSelect options -

EU-Konformitätserklärung (DoC)
Checkliste zur Prüfung der DoC.
50 CHF plus VATAdd to cart -

Importer Manual for Switzerland
Conformity without a certified QMS.
2'000 CHF plus VATSelect options -


IVDR Review
Read moreSupport checking your SOPs against the IVDR.
-


MDR Review
Read moreSupport checking your SOPs against the MDR.
-



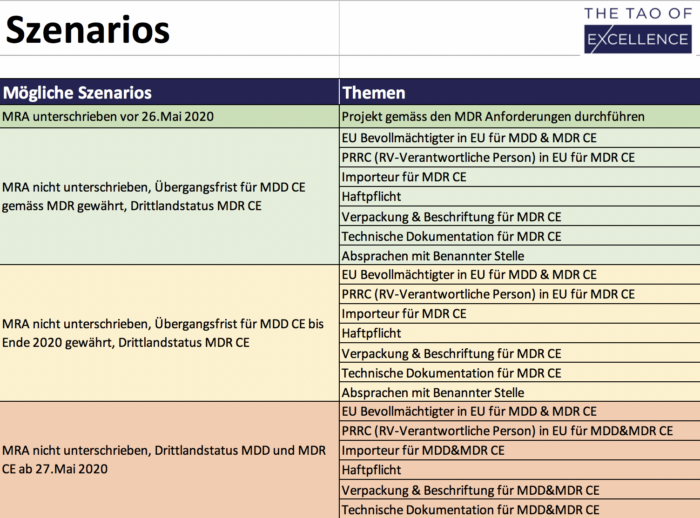
MRA Solutions Finder
Tool to optimize preparation for a missing MRA.
600 CHF plus VATAdd to cart -


RA / QA Membership
Read moreRegulatory and quality management support.
-



Regulatory document matrix for medical devices
For the EU and Swiss market
100 CHF plus VATAdd to cart
