RA / QA Membership Regulatory and quality management support.
Request a Quote
- Easy management and cost control giving you access to the right expertise as and when you need it.
- In-depth knowledge from proven experts specialising in medical devices, pharmaceuticals, and combination products.
- Complete regulatory and quality coverage including ISO 9001, ISO 13485, MDR, IVDR, and MDSAP.
- Practical advice, support and strategies for successful certification, audits and a strong QMS.
- Mentoring, leadership and training opportunities to optimise performance and maintain compliance and standards.
Description
Ideal for SMEs, our membership scheme allows you to easily access the full scope of regulatory and quality expertise at the highest level, as and when you need it, and with complete cost control. Reduce overall risk and improve efficiency, quality and performance by having this in-depth skillset on hand to ensure a smooth certification process, successful audits and continually compliant systems.
Benefits for all members
- All members can choose either a monthly or yearly subscription.
- All members are free to arrange their hours as needed.
- All members have access to our team of regulatory and quality experts and professional network.
- All yearly subscriptions will have a dedicated quality manager.
-
- Membership A
- 40 or 100 hours per month
- Second-party auditing
- Leadership coaching
- Interim management
- Member benefits
- Membership A
-
- Membership B
- 20 hours per month
- Second-party auditing
- Member benefits
- Membership B
-
- Membership C
- 10 hours per month
- Member benefits
- Membership C
What we offer:
- Clear, quick and agile solutions and strategies to secure CE certification.
- Hands-on expertise and sounding board for your questions and ideas.
- QMS set-up in line with ISO 13485 and MDR (EU) 2017/745 requirements.
- Manage all quality and regulatory related topics, including production support and hygiene expertise.
- Review and maintenance of your QMS, all technical documentation and SOPs in line with the related regulation and standards, ensuring continued compliance.
- Finding, selecting and managing all interactions with your chosen Notified Body.
- Post-market surveillance support to meet regulatory requirements.
- Mentoring or full support for your regulatory or quality teams to manage compliance and strengthen performance.
- Transparent service and complete cost control.
Request a Quote
You may also like…
-

MDR Review
Support checking your SOPs against the MDR.
Read more -

IVDR Review
Support checking your SOPs against the IVDR.
Read more -



Process Improvement 1
Level 1. Certified Six Sigma Yellow Belt Course.
1'420 CHF plus VATAdd to cart -




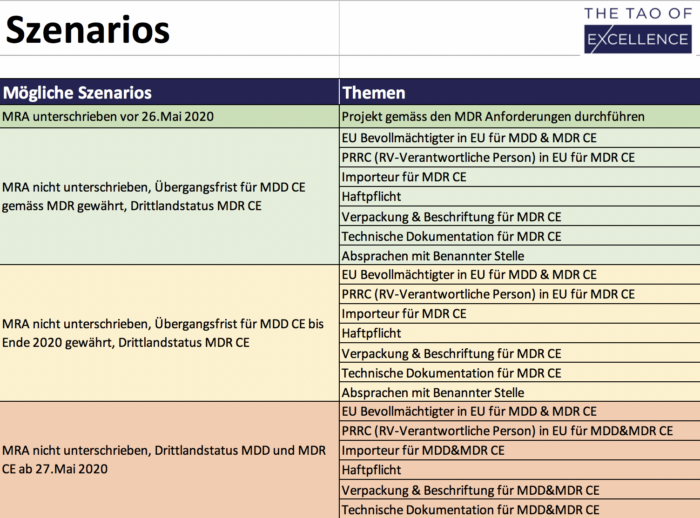
MRA Solutions Finder
Tool to optimize preparation for a missing MRA.
600 CHF plus VATAdd to cart