MDR Clinical EvaluationGap analysis tool for GCP compliance.
190 CHF plus VAT
- Downloadable excel file that is clear, accessible and easy-to-use.
- MDR and ISO 14155:2020 (GCP) compliance checklist of MDR requirements for clinical investigations.
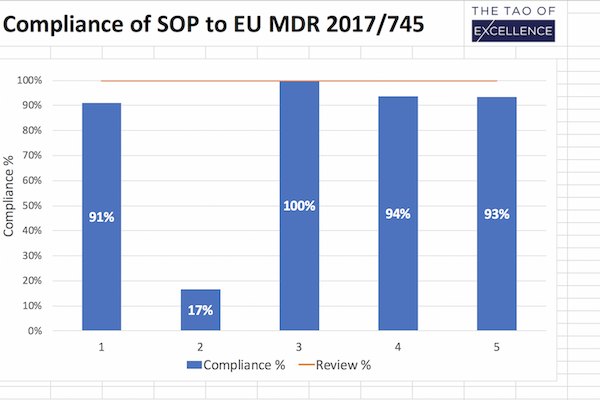
- Gap analysis tool to show you how compliant your SOPs are against individual standards and MDR requirements for clinical investigations.
- Identify an action plan using the checklist of tasks to complete to achieve compliance
- Review and track SOP compliance with a % table summary measuring how complete the update is.
- Maintain 100% compliance by using this tool after your SOP updates to ensure continued compliance.
Description
This downloadable excel document is built for quality, regulatory and process owner teams to ensure that your standard operating procedures (SOP) comply with the requirements set out for clinical investigations in the MDR (EU) 2017/745 and ISO 14155:2020 for Good Clinical Practice (ISO GCP). Designed as a unified, clear and easy-to-use document, this tool performs a gap analysis of the tasks you need to complete to comply with MDR requirements for clinical investigations. It is also a review and progress report you can use after your updates to ensure that your SOP continues to maintain 100% compliance.
Contact Form
You may also like…
-

MDR Review
Support checking your SOPs against the MDR.
Read more -


MDR Risk Management
Gap analysis tool for risk management compliance.
175 CHF plus VATAdd to cart -




MDR UDI
Gap analysis tool for UDI compliance.
155 CHF plus VATAdd to cart -




Post Market Surveillance
Gap analysis tool for PMS compliance.
155 CHF plus VATAdd to cart




