Market access to Switzerland
Medical devices and the regulatory framework
CE marking
(in compliance to MDR/MedDO or IVDR/IvDO)
We guide you on your journey
- From product idea,
- through development,
- setup of quality system,
- to complete technical documentation.
To your CE mark and beyond.
Swiss Authorized Representative (CH-REP)
We represent you as your CH-REP
- From compliance review
- through registration,
- market oversight and regulatory intelligence,
- to incident handling.
Full support for all things Switzerland.
Why choose us?
Your contact in Switzerland
- >20 year track record in Switzerland. We stand with our own name.
- Well connected network in Switzerland
- Regulatory intelligence for Switzerland (other markets available, such as EU, US, Australia)
- Strong expert quality, regulatory and clinical background
- Standardised CH-REP processes
- Contained, transparent costs
- Product liability insurance available
- Pure CH-REP function: no competition clause
- Fully registered with Swissmedic as CH-REP
Work with us
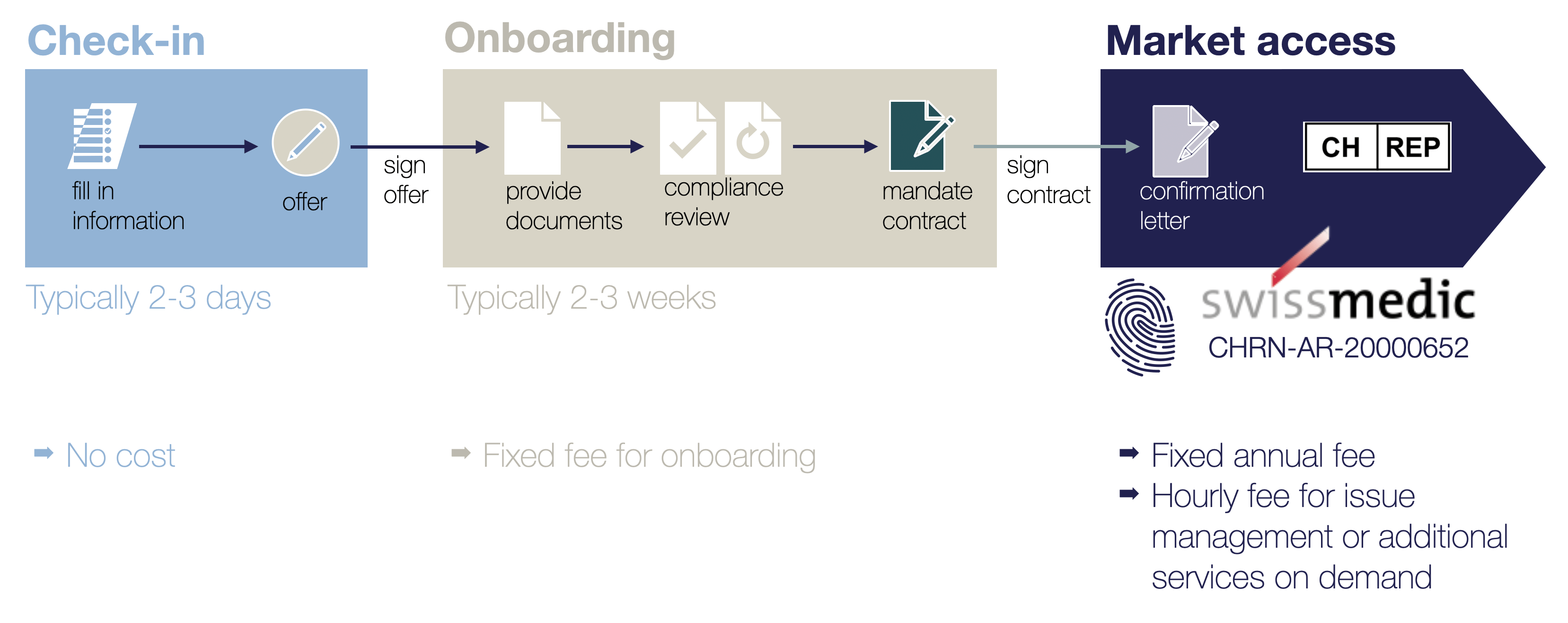
CH-REP onboarding process
How we support you
Medical devices for the Swiss market
- Regular updates on regulatory landscape in Switzerland
- Support tailored to your products
- Compliance review and gap analysis (ISO13485, MDSAP, MDR, IVDR, MedDO, IvDO, ISO9001 ao)
- Economic operator trainings (Manufacturers, Importers, Distributors, CH-REPs, PRRCs)
- Incident handling
- Communication with Swissmedic, Notified body, economic operators.
- On demand regulatory advise as an established partner
- Network acceleration within Switzerland
Get in Touch
Contact us for more information