Checklist for labelfor medical devices in EU and Switzerland.
175 CHF plus VAT
- Download of checklist for immediate use.
- MDR und MepV compliant checklist for manufacturers in EU and Switzerland.
- Conformity check through detailed listing of requirements.
- Basis for reviewing compliance of label with regulation.
- Customizable in Microsoft Word or Apple Pages format.
Description
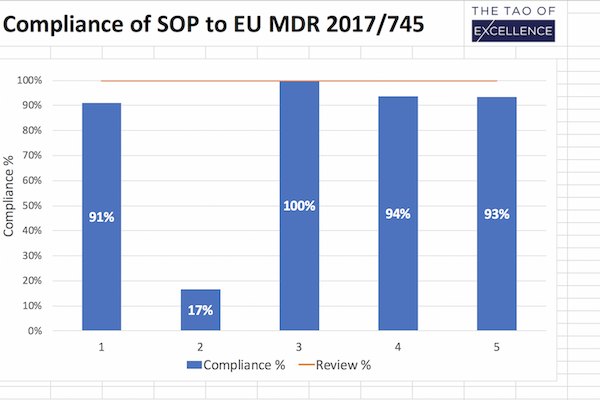
For manufacturers, importers, CH-REPs/EC-REPs of medical devices to ensure a label is designed to include all applicable information relevant to the type of the device. This checklist lists all requirements for the label according to MedDO (Swiss Medical Device Ordinance) and MDR (European Medical Device Regulation). It contains the full requirements, and the compliance is easy to check through the detailed checklist.
Language
German and English
Contact form